Aniline
Aniline is an organic compound with the formula C₆H₅NH₂. Consisting of a phenyl group attached to an amino group, aniline is the simplest aromatic amine. It is an industrially significant commodity chemical, as well as a versatile starting material for fine chemical synthesis
- Overview
- Application
- Spesification
Overview
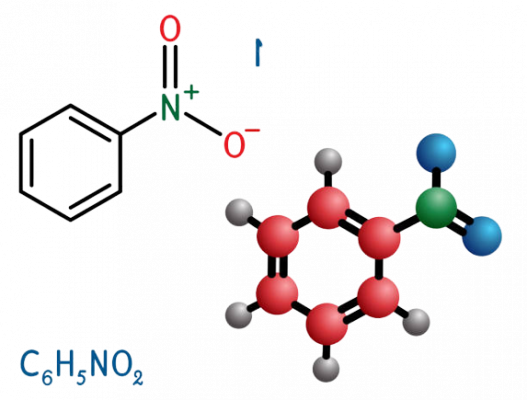
Aniline, phenylamine or aminobenzene is an organic compound with the formula C6H5NH2. It is an organic chemical compound, specifically an aryl amine, consisting of a phenyl group attached to an amino group. The chemical structure of aniline is shown at the right. It is now used mainly in the manufacture of polyurethane, although it previously was mainly used more for dyes and drugs.
PRODUCTION
Aniline is produced industrially in two steps from benzene:
First, benzene is heated with a concentrated mixture of nitric acid and sulfuric acid at 50 – 60 ºC, where one hydrogen atom is displaced to give nitrobenzene. In this nitration reaction, nitric acid first reacts with sulfuric acid giving the electrophile +NO2 which is attracted towards the ?-electron cloud of benzene0. The +NO2 electrophile attacks the carbon atom, displacing a proton H+ from that particular carbon atom. Nitration is thus called an electrophilic substitution reaction. Now a mixture of hydrogen gas and nitrobenzene vapors are heated at 600 ºC in presence of a nickel catalyst. This gives aniline by reduction. Aniline obtained here is in the pure state.
PROPERTIES
Aniline is oily and, although colourless, it slowly oxidizes and resinifies in air, giving the sample a red-brown tint. Like most volatile amines, it possesses a somewhat unpleasant odour of rotten fish, and also has a burning aromatic taste; it is a highly acrid poison. It ignites readily, burning with a smoky flame.
Chemically, aniline is a weak base. Aromatic amines such as aniline are generally much weaker bases than aliphatic amines. Aniline reacts with strong acids to form anilinium (or phenylammonium) ion (C6H5-NH3+), and reacts with acyl halides such as acetyl chloride to form amides. The amides formed from aniline are sometimes called anilides, for example CH3-CO-NH-C6H5 is acetanilide.
Application
Aniline (also called aminobenzene) is an oily, flammable liquid. It occurs naturally in some foods. It is produced in very large amounts (1 billion pounds in 1992) by seven companies in the United States. U.S. demand is likely to increase 3% to 4% per year for the next several years. The largest users of aniline are companies that make isocyanates, especially methyl diphenyl diisocyanate. Other companies use aniline to make pesticides, dyes, and rubber. Companies also use smaller amounts of aniline to make drugs, photographic chemicals, varnishes, and explosives.
Exposure to aniline can occur in the workplace or in the environment following releases to air, water, land, or groundwater. It enters the body when people breathe air or consume food or water contaminated with aniline. It can also be absorbed through skin contact. It does not remain in the body due to its breakdown and removal.
Originally the great commercial value of aniline was due to the readiness with which it yields, directly or indirectly, valuable dyestuffs. The discovery of mauve in 1856 by William Henry Perkin was the first of a series of dyestuffs which are now to be numbered by hundreds. Reference should be made to the articles dyeing, fuchsine, safranine, indulines, for more details on this subject.
In addition to its use as a precursor to dyestuffs, it is a starting-product for the manufacture of many drugs such as paracetamol (acetaminophen, Tylenol). It is used to stain neural RNA blue in the Nissl stain. Currently the largest market for aniline is preparation of methylene diphenyl diisocyanate (MDI), some 85% of aniline serving this market. Other uses include rubber processing chemicals (9%), herbicides (2%), and dyes and pigments (2%).
| PRODUCT IDENTIFICATION | ||
| CAS NO. | : | 62-53-3 |
| FORMULA | : | C6H5NH2 |
| MOL WT. | : | 93.12 |
| SYNONYMS | : | Aminobenzene; Benzenamine; Aminophen |
| PHYSICAL AND CHEMICAL PROPERTIES | ||
| PHYSICAL STATE | : | pale yellow semi-solid |
| MELTING POINT | : | 35 – 38 ºC |
| BOILING POINT | : | 276 ºC |
| SPECIFIC GRAVITY | : | 1.023 |
| AUTOIGNITION | : | 360 ºC |
| FLASH POINT | : | 120 ºC |
| NFPA RATINGS | : | Health: 2 Flammability: 1 Reactivity: 0 |
| STABILITY | : | Stable under ordinary conditions. Air, moisture sensitive. |
| SALES SPECIFICATION | ||
| APPEARANCE | : | pale yellow semi-solid |
| CONTENT | : | 98.0% min |
| TRANSPORTATION PACKING | : | 200kgs in drum |



